The HiFAE trial

Home high-flow therapy (HFT) has been shown to reduce acute exacerbation rates and hospital admissions in small studies of patients with COPD.1,2 HiFAE will bring a new level of evidence on the benefits of home HFT in a large cohort of severe COPD patients.
Study objectives
The main objective is to evaluate the efficacy of home HFT with oxygen for reducing severe exacerbations versus standard long-term oxygen therapy (LTOT) in patients previously admitted to hospital for a COPD severe exacerbation. The trial will also evaluate the benefit(s) of home HFT with oxygen on quality of life, respiratory function, exercise capacity, as well as cost effectiveness, compliance and more.
Patient population
Main inclusion criteria*:
• Patients with COPD admitted to hospital for a severe COPD exacerbation
• Patients with an indication for LTOT
Main exclusion criteria*:
• Treatment with non-invasive ventilation or continuous positive airway pressure
• BMI >35 kg/m2
• Acute COVID-19
Study details
Study type
Prospective, multicentre, open-label, parallel-group, randomised, controlled trial
Primary investigators
Dr Maxime Patout (La Pitié Salpetrière Hospital) and Prof. Antoine Cuvelier (University Hospital Rouen)
Sponsor
University Hospital Rouen – Hospital Clinical Research Programme of the French Ministry for Solidarity and Health**.
Study design
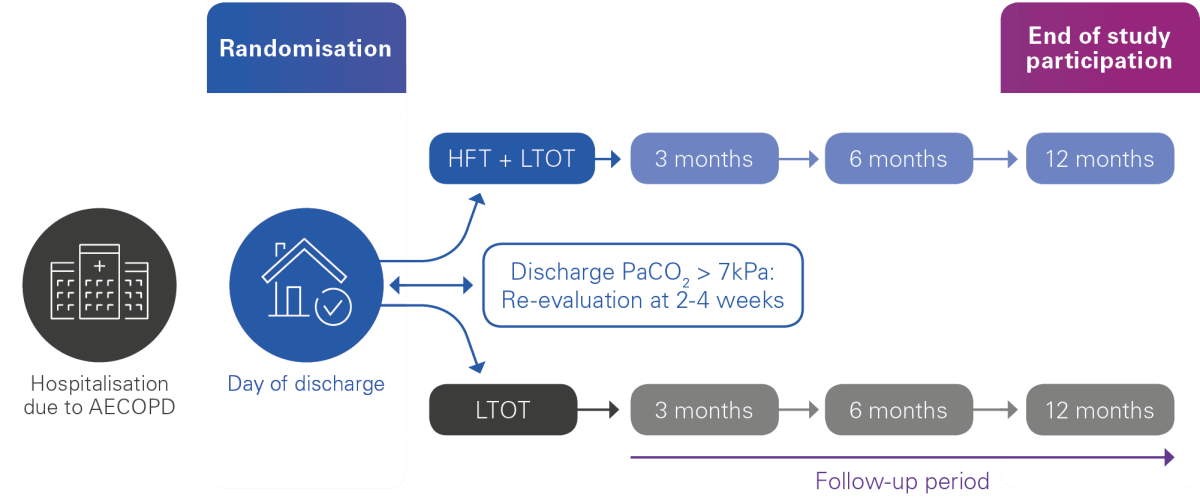
406 patients randomised into two groups:
The intervention group: Home HFT + LTOT
The control group: LTOT alone
The enrolment period will last 36 months, with a follow-up period of 12 months, for a total study duration of 48 months.
The study endpoints
Primary endpoint:
Time to first hospital admission for AECOPD or death
Secondary endpoints:
The following endpoints will be assessed at 1 year:
- Change from baseline in health-related quality of life
- Change from baseline in gas exchange (PaO2, PaCO2)
- Change from baseline in FEV1 (spirometry)
- Change from baseline in distance walked during the 6-minute walk test
- Clinical course of the disease: all-cause admission-free survival at 1 year, overall survival, exacerbation frequency
- Cost-effectiveness: total costs, cost-utility
- Compliance and tolerance of HFT in the home setting
- Improvement of chronic hypoxemia during follow-up
18 centres

All the news, all in one place
Have you visited our new clinical news page?
Don’t miss out on the latest respiratory research articles and news.
Support for investigator initiated research
ResMed believes in the need to support ethical, independent clinical research, conducted by qualified third-party investigators.
Learn more: Home HFT for COPD

Home high-flow therapy for COPD
Learn how HFT could be indicated for home use in COPD patients experiencing secretion management issues.

Webinars on home high-flow therapy
Experts explain the evidence for using HFT in different indications, discuss current research gaps and explore the potential benefits of this emerging therapy.
This content is intended for health professionals only
* Please see https://clinicaltrials.gov/ct2/show/NCT05196698 for further information
** Programme Hospitalier de Recherche Clinique (PHRC) du Ministère des solidarités et de la santé. Please see https://sante.gouv.fr/systeme-de-sante/innovation-et-recherche/l-innovation-et-la-recherche-clinique/appels-a-projets/article/programme-hospitalier-de-recherche-clinique-phrc for further information
- Storgaard LH, et al. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis 2018;13:1195-1205. DOI : 10.2147/COPD.S159666
- Rea H, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med 2010;104:525-533. DOI : 10.1016/j.rmed.2009.12.016