The HiLOT trial

Home high-flow therapy (HFT) has been shown to reduce acute exacerbation rates and hospital admissions in patients with chronic pulmonary obstructive disease (COPD)1,2.
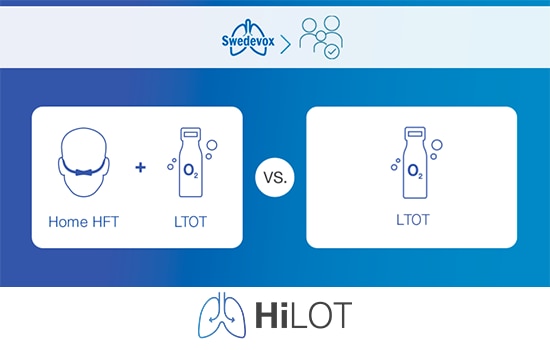
With the need to grow the level of evidence on the benefits of home HFT, HiLOT aims to evaluate the effect of using home HFT with long-term oxygen therapy (LTOT) during one year in people with COPD or interstitial lung disease (ILD), vs using LTOT alone.
Study objectives
The main objective is to evaluate the impact of home HFT with LTOT on mortality and hospitalisations in patients with COPD. The trial will also evaluate the benefit(s) of home HFT with LTOT on quality of life and symptoms and assess the cost effectiveness, compliance and tolerance of the therapy.
Patient population

Main inclusion criteria*:
• COPD patients on LTOT
• ILD patients on LTOT
• BMI <35kg/m2
• > 40 years
Main exclusion criteria*:
• Current treatment with HFT, non-invasive ventilation or CPAP
• BMI > 35kg/m2
• Hospitalisation in the last 2 weeks
Study details

Study type
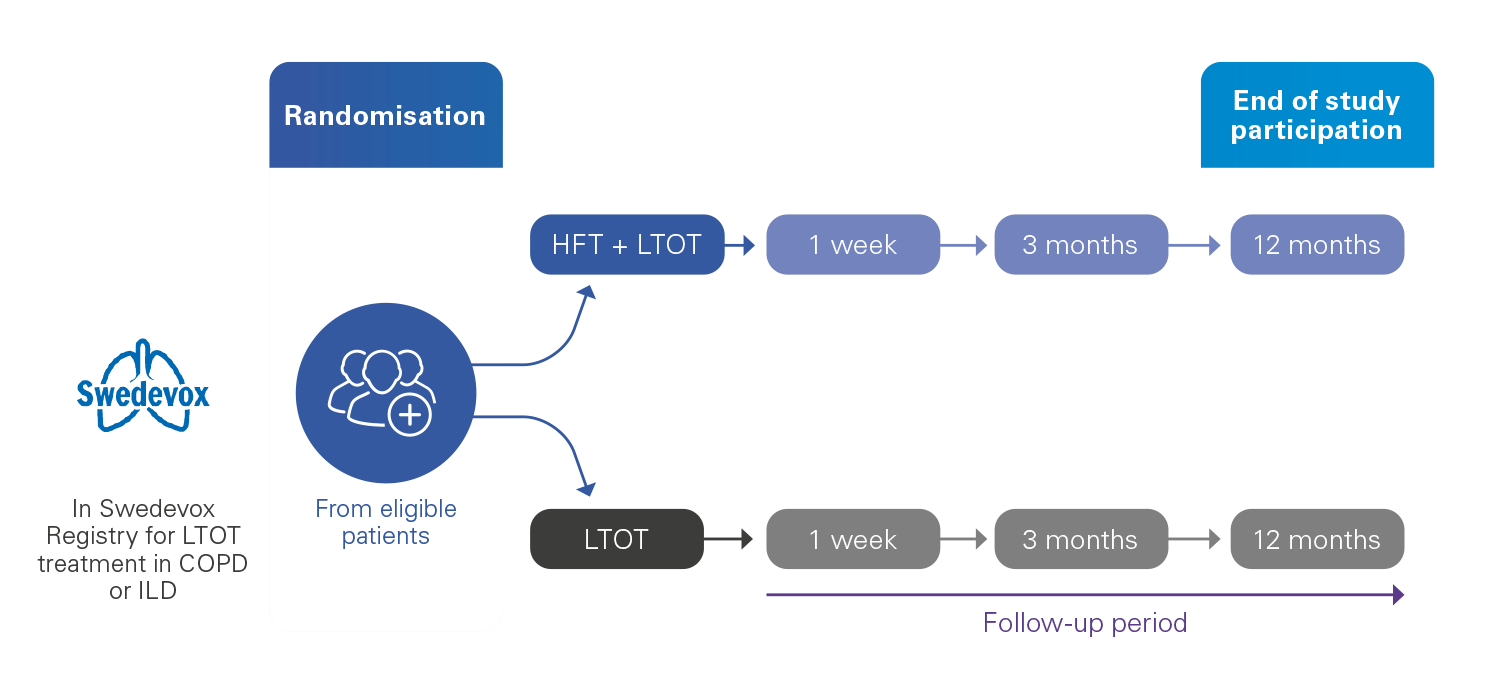
Prospective, multicentre, registry-based, randomised controlled trial with parallel groups.

Primary investigators
Assoc. Professor Magnus Ekström, Department Respiratory Medicine, Skåne University Hospital Lund, Sweden
Sponsor
Skåne University Hospital Lund, Sweden
Study design
310** patients randomised in two groups:
The intervention group: Home HFT + LTOT
The control group: LTOT alone
The enrolment period will last 36 months, with a follow-up period of 12 months, for a total study duration of 48 months.
The study endpoints
Primary endpoint:
Time to first hospitalisation or all-cause death in the year following randomisation in COPD patients.
Secondary endpoints include:
• Symptoms
• Health-related quality of life
• Hospitalisations
• Exacerbations
• Cost effectiveness
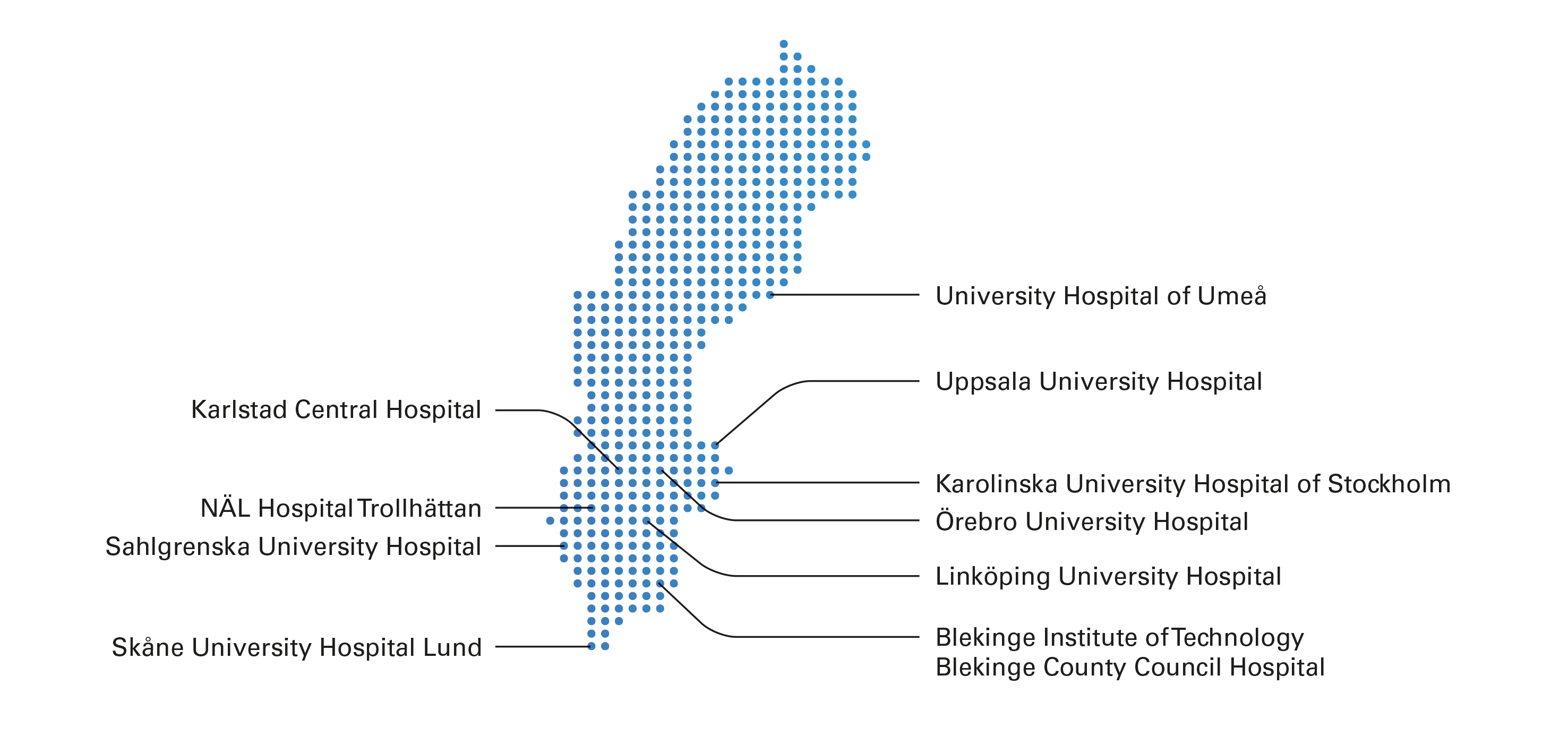
Taking place across 10 centres
Sign up for our clinical newsletter
Sign up to receive clinical updates about COPD, high-flow therapy, non-invasive ventilation, remote monitoring and more. You can also visit our Clinical News page for a selection of the latest research in these areas.
Support for investigator-initiated research
ResMed believes in the need to support ethical, independent clinical research, conducted by qualified third-party investigators.
Learn more: Home HFT for COPD

The expert’s voice
In this episode of ResMed’s OnAir podcast, Dr Maxime Patout explores the ever-evolving world of high-flow therapy, and its implications for at-home care.

Home high-flow therapy for COPD
Learn how HFT could be indicated for home use in COPD patients experiencing secretion management issues.

Webinars on home high-flow therapy
Experts explain the evidence for using HFT in different indications, discuss current research gaps and explore the potential benefits of this emerging therapy.
This content is intended for health professionals only
*Please see https://www.clinicaltrials.gov/study/NCT06247397 for further information
**270 COPD patients and 40 ILD patients
- Storgaard LH, et al. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis 2018;13:1195-1205. DOI : 10.2147/COPD.S159666
- Rea H, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med 2010;104:525-533. DOI : 10.1016/j.rmed.2009.12.016V
Content last updated: 04/2024