Information regarding use of ResMed masks with magnetic clips
Last updated: November 9, 2022
Please check back for updates
Patient safety is always ResMed’s first priority. We have created this site to keep you informed about using ResMed masks with magnetic clips. ResMed masks are safe to use when you follow ResMed’s instructions for use, regulatory guidance applicable in your country, and any available information provided by your doctor, equipment provider, or the manufacturers of other devices you may use1.
Our instructions are found in the user guide applicable to your mask. Our instructions generally provide that for patients who have active or medical implants2, such as a pacemaker, defibrillator, magnetic cerebrospinal fluid (CSF) shunt valve or cochlear implants, it is recommended that the headgear and frame be kept at least 2 inches (50 mm) away from any active medical implants to avoid possible effects from localized magnetic fields.
In response to changes in technologies and recent post market information, some regulators in other parts of the world are revising recommendations around the use of magnets with some implantable devices.
We are evaluating these developments, including a recent safety notification by a competitor, to determine if we should enhance any aspect of our labelling and instructions for use, or our education to equipment providers and patients on the safe use of ResMed masks with magnets. If you have any questions or concerns, please read our FAQs below and consult with your doctor or equipment provider.
Does this apply to me?

This information pertains to a limited number of CPAP therapy users who have active medical implants such as a pacemaker, defibrillator, magnetic cerebrospinal fluid (CSF) shunt valve or cochlear implants. As stated in ResMed’s instructions for use, masks with magnets are contraindicated (not appropriate) for patients with the following pre-existing conditions:
- A metallic hemostatic clip implanted in your head to repair an aneurysm
- Metallic splinters in one or both eyes following a penetrating eye injury
If you have questions about your specific situation, please consult with your doctor or equipment provider.
Which ResMed masks contain magnets?
Use the links below to find more information on each ResMed mask that features magnetic clips.
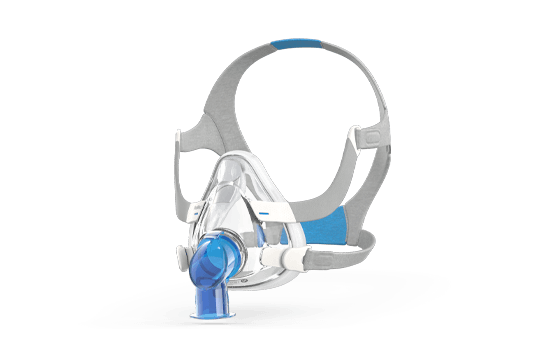
Full-face masks*
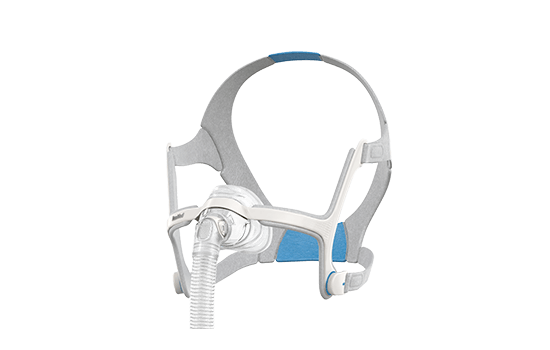
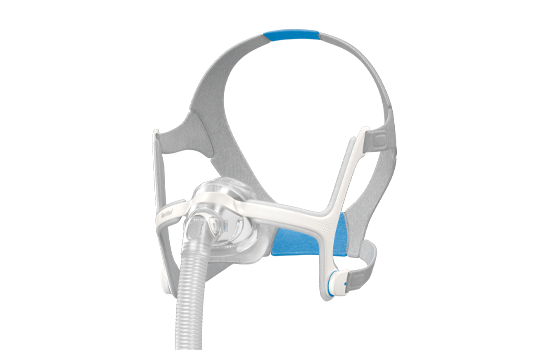
Nasal masks*
General FAQs
Yes. Magnets are used in the lower headgear straps and frame of select ResMed masks, and the magnetic strength is within the limits set by the International Commission for Non-Ionizing Radiation Protection for general public use1.
All ResMed masks that include magnets come with labelling and instructions to users about the use of magnets near metallic implants and other medical devices.
In response to changes in technologies and recent post market information, some regulators in other parts of the world are revising recommendations around the use of magnets with some implantable devices.
In light of this, and a recent safety notification by a competitor, we are evaluating this labelling and instructions to determine if changes could improve education to providers and patients on the safe use of ResMed masks with magnets, reinforcing patient safety.
- The magnet strength is specified to be less than 400mT, but typically measures within 160mT to 300mT. These values remain within the limits set by the International Commission of Non-Ionizing Radiation Protection for use of magnets in this setting.
- Talk to your doctor or equipment provider about any risks associated with using a mask with magnets.
The following ResMed masks use magnets:
Full-face masks*: AirFit F20, AirFit F30, AirFit F30i, AirTouch F20, AirFit F20 Non-vented
Nasal masks*: AirFit N20, AirTouch N20, AirFit N10
ResMed follows established design, verification, and validation processes to ensure that its products are safe to use and comply with all applicable standards at that time. ResMed also has processes to evaluate and react to post-market information that becomes available.
Patient safety is and has always been ResMed’s top priority. All ResMed masks are safe when used as directed. All ResMed masks that include magnets come with labelling and instructions to users about the use of magnets near other medical devices.
In response to changes in technologies and recent post market information, some regulators in other parts of the world are revising recommendations around the use of magnets with some implantable devices.
In light of this, and a recent safety notification by a competitor, we are assessing ResMed masks and associated product labelling to determine if changes could improve education to providers and patients on the safe use of ResMed masks with magnets, reinforcing patient safety.
In the interim, we recommend users read all labelling and instructions that come with each ResMed mask that includes magnets and talk to their clinician about any risks before using masks that have magnets if they have an implanted medical device.
FAQs for CPAP users
Environmental policy statement
ResMed is committed to working with our employees, suppliers and customers to protect the environment and in doing so to systematically reduce our costs. We aim to achieve this by:
ResMed masks are safe when following ResMed’s instructions for use. All ResMed masks that include magnets come with labelling and instructions about using magnets near other medical devices. We recommend following the instructions in your mask user guide, regulatory guidance applicable in your country, and to talk to your doctor about any risks associated with using a mask with magnets.
The following ResMed masks feature magnetic clips:
- Full-face masks*: AirFit F20, AirFit F30, AirFit F30i, AirTouch F20, AirFit F20 Non-vented (NV)
- Nasal masks*: AirFit N20, AirTouch N20, AirFit N10
Where do I find the model of my mask?
-
AirFit F20/AirFit F20 NV: Bottom of the cushion, directly below the elbow. AirFit F20 non-vented has a blue non-vented elbow
-
AirFit F30: Top of top headgear strap or on the bottom of the frame, directly below the elbow
-
AirFit F30i: Back of headgear, bottom of the cushion
-
AirTouch F20: Bottom of the cushion, directly below the elbow
-
AirFit N20: Bottom of the cushion, directly below the elbow
-
AirTouch N20: Bottom of the cushion, directly below the elbow
-
AirFit N10: Frame’s arm, back of headgear
Consult your physician or equipment provider for more information on which mask may be right for you.
The following ResMed masks do not use magnets:
- Full-face masks*: Quattro Air, Mirage Quattro, Quattro Air Non-vented
- Nasal masks*: AirFit N30, AirFit N30i, Mirage FX, AirFit N20 Classic
- Nasal pillows*: AirFit P10, AirFit P30i, Swift FX
- There are various types of active medical devices, including pacemakers, defibrillators, magnetic cerebrospinal fluid (CSF) shunt valves or cochlear implants.
- Please note that this list is not inclusive of all medical implants. If you have any implanted medical device in the head and/or chest region, please consult your doctor or implanted device manufacturer.
No. Mobile phones have an alternating magnetic field. The magnets on ResMed’s headgear clips have a static magnetic field and meet current safety guidelines1.
FAQs for healthcare professionals
Environmental policy statement
ResMed is committed to working with our employees, suppliers and customers to protect the environment and in doing so to systematically reduce our costs. We aim to achieve this by:
Consult your ResMed sales representative for recommendations around mask crosswalk information.
The following ResMed masks do not use magnets:
- Full-face masks*: Quattro Air, Mirage Quattro, Quattro Air Non-vented
- Nasal masks*: AirFit N30, AirFit N30i, Mirage FX, AirFit N20 Classic
- Nasal pillows*: AirFit P10, AirFit P30i, Swift FX
* Availability of these masks may vary depending on the country.
References:
1 – The guidelines for public exposure to static magnetic fields are established by the International Commission for Non-Ionizing Radiation Protection (ICNIRP) and are recognized by the World Health Organization (WHO).
2 – Please note that this list is not inclusive of all medical implants. If you have any implanted medical device in the head and/or chest region, please consult your physician or implanted device manufacturer.